Abstract
Background
Chimeric antigen receptor T (CAR T) cells targeting CD19 have clinical activity against relapsed or refractory (r/r) non-Hodgkin lymphoma (NHL), with high response rates. However, approximately a third of patients fail to respond to CAR T cells and more than half will present disease progression within one year. Circulating tumor DNA (ctDNA) is emerging as a powerful tool for prediction of response in hematologic malignancies. Here, we present the results of ctDNA analysis of 28 patients participating in a phase I clinical trial of point of care manufactured antiCD19 CAR T cells with TNFRSF19 transmembrane domain using CliniMACS Prodigy device (Caimi PF, et al. Blood 2021; 138(S1):3833) (NCT03434769).
Methods
Twenty-eight relapsed or refractory NHL patients treated with CAR T cells were included in the ctDNA analysis. Blood samples were collected at baseline (prior to lymphodepletion), on the day of infusion (T = 0) and 30 days after CAR T cell infusion and profiled using the CAPP-Seq strategy (Rossi, 2017). A targeted resequencing panel optimized to include the coding exons and splice sites of 154 genes (370 Kb) recurrently mutated in B cell NHL were used on cfDNA from plasma and on paired germline DNA isolated from paired peripheral blood mononuclear cells. Sequencing was performed using the Nextseq 550 platform (Illumina) to obtain a depth of coverage >2000x in >80% of the target region in all samples. A stringent and completely automated bioinformatic pipeline was applied to call non-synonymous somatic mutations, using the somatic function of VarScan2. Disease response was measured on day 30 and 90 with PET/CT scan. Progression free survival (PFS) and overall survival (OS) were measured from the time of CAR T cell infusion and estimated using Kaplan-Meier analysis. Statistical analyses were done using R and its packages. Comparisons between groups were done using Fisher exact test between categorical variables, optimal ctDNA cutpoints for categorical endpoints were done using the cutpointr R package, and using the maximally selected rank statistics for time to event endpoints.
Results
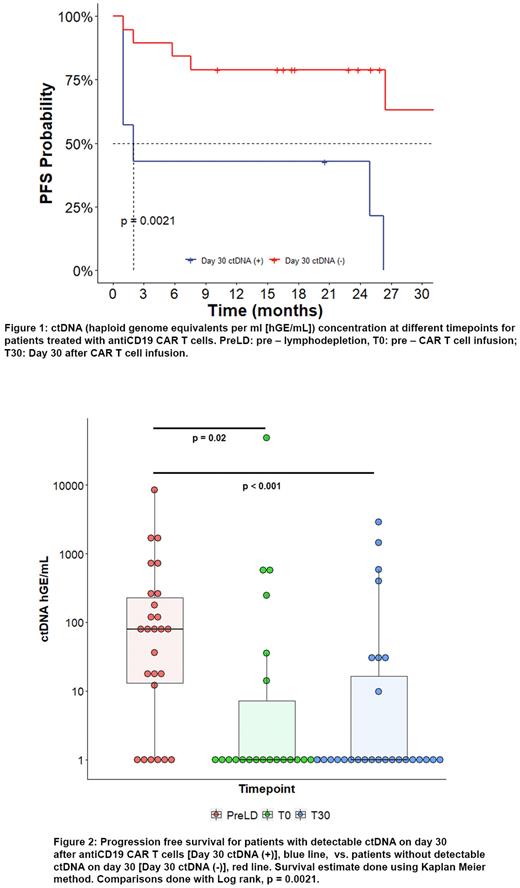
75 plasma samples were available for analysis (T-6, n = 26; T0, n = 22; T30, n = 27). The median ctDNA load reported as haploid genome equivalents per ml (hGE/mL) was 75.81 at T-6 (range: 0-8373). There was a statistically significant decrease in ctDNA load on T0 (0,33 hGE/mL [range: 0-48126], p = 0.02) and T+30 (0,38 hGE/ml [range: 0-2855], p < 0.001)(Figure 1). Six patients (23%) had undetectable ctDNA on T-6, despite having measurable disease.
The optimal cutoff value for T-6 ctDNA was determined at 250 hGE/mL, patients with lower values (20/26) had higher rates of complete response (CR) (95% vs. 33%, p < 0.01) as well as longer median PFS (26 vs. 2 months, p < 0.001) and OS (NR vs. 20 months, p <0.01). We did not find an association between higher T-6 ctDNA levels and CRS or ICANS. Genes recurrently affected by non-synonymous somatic variants in 26 patients at T-6 were BCL2 and KMT2D (27%), IGLL5 (23%), BCL6 and CARD11 (19%), MYC and TP53 (15%).
Lymphodepletive chemotherapy resulted in a decrease in ctDNA in 17/20 patients with paired samples, 13 patients decreased ≥1 log and 8 by ≥ 2 log. Sixteen patients had undetectable ctDNA levels at T0, which includes 9 patients with previous measurable ctDNA. Patients with ctDNA under 10 hGE/ml had higher CR rates (88% vs. 33%, p = 0.03) as well as longer median PFS (26 vs. 1 month, p = 0.03) and OS (NR vs. 13 months, p = 0.005).
On day T30, 21/26 patients with paired preCAR T samples had decreased ctDNA by >1 log, 19 (70%) had undetectable levels. Among patients with undetectable ctDNA on day 30, best response was CR for 18 (94%), 4 of whom had PR on PET/CT on day 30 and went on to convert to CR. Patients with undetectable ctDNA had longer median PFS (37 vs. 2 months, p = 0.002) (Figure 2) and OS (NR vs. 13 months, p < 0.001).
Conclusions
Detection of ctDNA in r/r NHL patients treated with CAR T cells has predictive value at different timepoints. Lower ctDNA burden prior to lymphodepletion is associated with improved outcomes, likely a reflection of lower disease burden. In addition, ctDNA concentration decreases both after lymphodepletive chemotherapy and after CAR T cells correlate with disease response, PFS and OS. Mutational profiling of ctDNA can provide information regarding recurrent mutations in cases with and without disease response to CAR T cells.
Disclosures
Caimi:Takeda: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Honoraria; ADC Therapeutics: Honoraria, Research Funding; Janssen: Consultancy; GenMab: Honoraria; Novartis: Consultancy; Kite: Consultancy; Genentech: Consultancy; Incyte: Consultancy; BMS: Honoraria. Ghobadi:Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Atara: Consultancy; Amgen: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy; Celgene: Consultancy; Wugen Inc: Consultancy; Genentech: Research Funding. Metheny:Gamida: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. de Lima:Celgene: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Incyte: Consultancy; Amgen: Consultancy. Carlo-Stella:Merck Sharp & Dohme: Honoraria; ADC Therapeutics: Honoraria, Other: Consultancy/Advisory, Research Funding; Roche: Other: Consultancy/Advisory, Research Funding; Bristol Myers Squibb: Honoraria; Sanofi: Other: Consultancy/Advisory, Research Funding; Incyte: Honoraria; Novartis: Honoraria; Janssen Oncology: Honoraria; AstraZeneca: Honoraria; Takeda: Honoraria; Celgene/Bristol Myers Squibb: Other: Consultancy/Advisory; Karyopharm Therapeutics: Other: Consultancy/Advisory; Scenic Biotech: Other: Consultancy/Advisory.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal